Biocide approval General consulting and notification and approval services
for the manufacture/importation of biocidal substances for use in biocidal products.
Biocide approval
- K-REACH
- K-BPR compliance
- Diagnosis of workplaces under CCA
- Chemical management IT solution
Overview
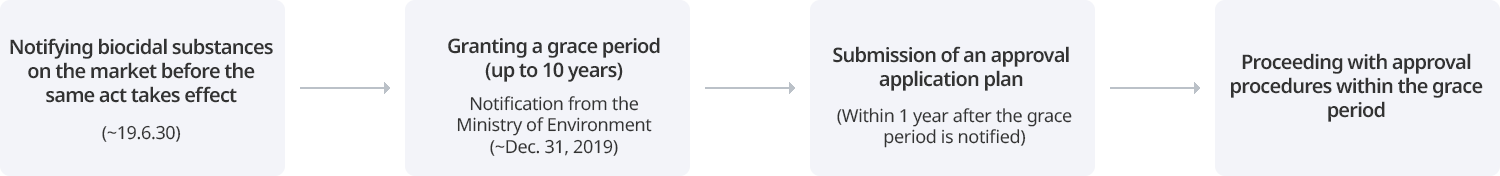
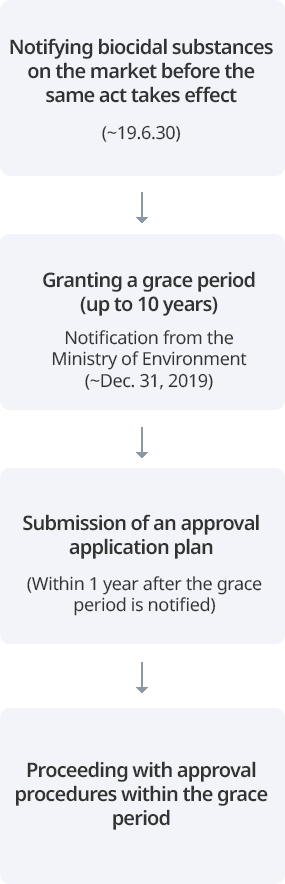
For existing biocidal substances, notification is required to obtain a grace period for approval. After notification, biocidal substances are approved sequentially by product type. New biocidal substances must be approved prior to manufacturing and importation without a grace period.
Notification of existing biocidal substances
A person who intends to manufacture or import an existing biocidal substance (a biocidal substance contained in a biocidal product distributed in Korea until December 31, 2018) may be granted a grace period for approval of the substance if the notification of the existing biocidal substance is made to the Minister of Environment within the specified period (January 1, 2019 to June 30, 2019). When an existing biocidal substance is notified, the type of product in which the substance is used must be declared as well, and depending on the type of product, a grace period of up to 10 years will be granted. During this period, existing biocidal substances can be manufactured and imported without approval.
There are 15 types of products in which biocidal substances regulated under the Korean Chemical Safety Act are used.
| Category | Type of biocidal products | Definitions |
|---|---|---|
| Disinfectants | Germicides | Sterilization, disinfection, antimicrobial, etc. |
| Algicides | To inhibit the growth of alga in water and exterminate them. | |
| Pesticides | Rodenticides | To remove rodents such as rats |
| Control of other vertebrates | To remove harmful vertebrates, except rodents | |
| Insecticides | To remove insects | |
| Control of other invertebrates | To remove harmful invertebrates, except insects | |
| Repellents | To neutralize or suppress harmful organisms by repellent methods | |
| Preservatives | Product preservation | To store and preserve products to ensure product shelf life |
| Product surface treatment | To preserve product surface coatings to protect the initial properties of the product surface | |
| Textiles and leather | For preserving textiles, leather, and rubber | |
| Wood | Preserving wood, wood products | |
| Building materials | Preserving building materials except wood | |
| Materials and equipment | Liquids, fluid preservation | |
| Cadavers and taxidermy | Human or animal carcasses, partially preserved | |
| Others | Antifouling agents for ships and underwater facilities | Prevent harmful organisms from growing in ships, aquaculture equipment, and underwater structures |
Submission of a substance approval application plan
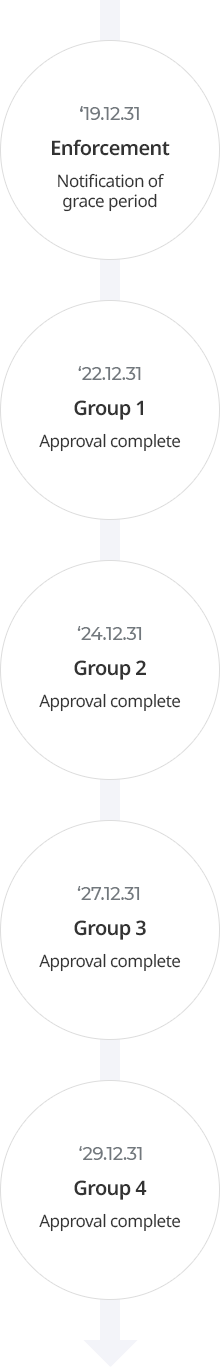
A person who has notified an existing biocidal substance must submit a substance approval application plan (a plan on the procedure and method of preparing substance approval application materials) within one year from the date on which the notified substance is designated and notified as an existing biocidal substance. A person who submits a substance approval application plan must properly prepare approval materials according to the plan.
| Classification | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|
| Type of biocidal products | Germicides, algicides, rodenticides, insecticides, repellents | Preservatives for wood, control of other vertebrates and invertebrates | Preservatives for product preservation, preservatives for product surface treatment, preservatives for textiles and leathers | Preservatives for building materials, preservatives for materials and equipment, preservatives for cadavers and taxidermy, antifouling agents for ships and underwater facilities |

Submission data for approval
A person who intends to manufacture or import a biocidal substance for use in a biocidal product must prepare and submit data to obtain approval from the Minister of Environment. The effective period for biocidal substances is set within 10 years depending on the characteristics of the substance, and re-approval is required before the expiration date.
| NO | Submission Data | Statutory Statement | Testing | |
|---|---|---|---|---|
| Law | Presidential Decree | |||
| 1 | Applicant's general information | O | X | |
| 2 | Substance identification information (molecular formula, chemical composition, etc.) | O | O | |
| 3 | Physical, chemical or biological properties (including physical hazards) | O | O | |
| 4 | Classification and labeling | O | X | |
| 5 | Usage and exposure information (including type of a biocidal product) | O | X | |
| 6 | Manufacturing process, etc. | O | X | |
| 7 | Efficacy and effectiveness | O | O | |
| 8 | Health hazards | O | O | |
| 9 | Environmental hazards | O | O | |
| 10 | Handling Precautions and Disposal | O | X | |
| 11 | Domestic and international use and regulatory information | O | X | |
| 12 | Comprehensive data on stability (including risk assessment) | O | X | |
| 13 | Applications of biocidal products in which biocidal substances may be used | O | X | |
Our services
Notification of existing biocidal substances
Establishing and writing substance equivalence data for biocidal substances
Approval of biocidal substances
Operation of consortiums
Data gap analysis and communication with international data owners
Writing CSRs
Arranging and scheduling tests
Responding to regulatory compliance requests from customers