Chemical registrationWe provide professional chemical registration services,
from operating consortiums to obtaining hazard test data and preparing registration dossier.
Chemical registration
- K-REACH
- K-BPR compliance
- Diagnosis of workplaces under CCA
- Chemical management IT solution
Overview
According to the K-REACH, registration must be completed for new chemicals (non-phase-in substances) used 0.1 ton or more and existing chemicals (phase-in substances) used 1 ton or more. If a chemical is manufactured or imported without registration, the law stipulates that the manufacture, import, use, and sale can be restricted.
We offer specialized services for chemical registration, encompassing operations within consortiums, acquisition of hazard test data, and compilation of registration dossiers.
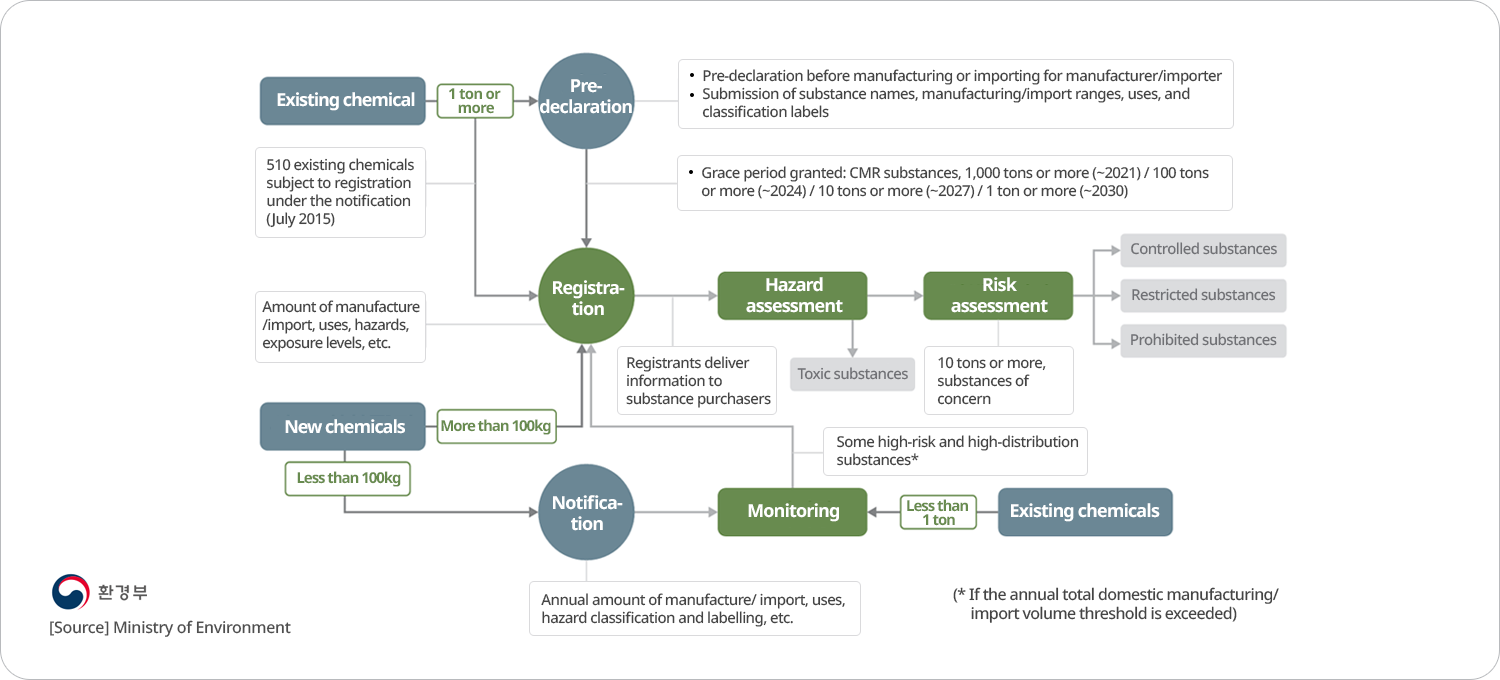
Chemicals subject to and exempt from registration
| Chemicals subject to registration | 0.1 ton or more of new chemicals or 1 ton or more of existing chemicals per year (1 ton or more of non-phase-in and phase-in starting from 2025) |
|---|---|
| Calculation of tonnage | Expected manufacturing/import volume from January 1 through December 31 of the year at the time of registration application |
| Unconditional exemption |
- Chemicals imported in machinery - Chemicals imported together with machinery or devices used for trial operation - Chemicals contained in a product that performs a certain function in a specific solid form and does not leak out in the course of use |
| Conditional exemption |
- Chemicals intended to be manufactured or imported for scientific experimentation, analysis, or research, such as reagents (one-time only) - Chemicals intended to be manufactured or imported for research and development (R&D planning units) - Polymers of low concern (one-time only) - Surface-treating substances or substances subject to surface treatment which are registered or pre-declared as new substances and are intended to be manufactured or imported (one-time only) - Substances manufactured and imported for full export to foreign countries or substances manufactured and imported for the manufacture of other chemicals for full export to foreign countries (annually) - Non-isolated intermediates - Isolated intermediates meeting certain requirements (one-time only) |
Dossier by registration type
| Registration type | Dossier |
|---|---|
| Small-volume registration (less than 100 kg, but less than 1 ton will be declared starting in 2025) |
1. Name, address, and representative of the person who intends to manufacture or import chemicals 2. Chemical name, molecular formula, structural formula, identification number, trade name, purity, name and content of identified impurities and by-products 3. Use of the chemical substance 4. Other data prescribed by the MOE Decree |
| Registration of isolated intermediates |
1. Name, address, and representative of the person who intends to manufacture or import chemicals 2. Chemical name, molecular formula, structural formula, identification number, trade name, purity, name and content of identified impurities and by-products 3. Use of the chemical substance 4. Classification and labeling of chemicals 5. Physical and chemical properties of chemicals |
| General registration and polymer registration |
1. Physical and chemical properties (1 copy) 2. Health and environmental hazards (1 copy) 3. CSR (1 copy) 4. Guidelines for safe use (1 copy) 5. One document proving consignment, such as a copy of the consignment agreement (if the chemicals were manufactured on consignment; submit only if requested by the consignor.) 6. Types of registration application materials that are omitted pursuant to Article 12 of the MOE Decree and one copy of the supporting documents 7. One copy of study protocol 8. One copy of CSR including exposure scenarios 9. Notification of appointment of foreign manufacturer (submit only if a foreign manufacturer was appointed under Article 38 of the Act.) 10. For polymeric compounds, data on number-average molecular weight, residual monomers, etc. (for polymer registration only) 11. For polymer compounds, data on number-average molecular weight and residual monomers (applicable only for polymer registration) |
Our services
Joint registration of existing chemicals and operation of consortiums
Registration and notification of new chemicals
Registration and notification of changes to chemical substances
Data gap analysis for registration
Negotiation for purchase of overseas data and operation of LOA shops
Production of test data and survey on uses of downstream users
Preparation of CSRs
Submission of data and response to supplemental requests
Features and benefits
01
- Most registrations in K-REACH 1st place
- No. 1 company in Korea for registration and consulting on K-REACH
- Know-how in compliance diagnosis, assessment, and prescription consulting for all provisions of the K-REACH
02
- Collaboration with over 100 experts
- Collaborate with the best environmental experts on chemical substance and product toxicity and risk
- Operate consortiums for joint registration and organize a collaborative response system with each expert team
03
- A large number of related DB and IT solutions
- Database of regulatory information for 220,000 chemicals worldwide
- Provide database in 33 languages and real-time information from an updated chemical regulation database
- A wide range of relevant IT solutions
Workflow